Blood tests could revolutionize diagnostics for rare hereditary disease
Isabelle Weinhofer from the Center for Brain Research at the Medical University of Vienna has to go into great detail when explaining her research, since the disease with the difficult name X-linked adrenoleukodystrophy, or X-ALD for short, is not only largely unknown, it is also challenging when it comes to medical, diagnostic and therapeutic aspects.
X-ALD is a genetic hereditary disease. With an incidence of 1:14700 in the total population, it is a rare disease. For Austria, this translates to around 620 affected individuals. Many of these remain undiagnosed, however.
Due to mutations in a specific gene, the ABCD1 gene, the body of patients is unable to break down the self-produced saturated very long-chain fatty acids in the same way as in healthy people. Accumulating in the tissue, especially in the brain and spinal cord, the lipids trigger toxic inflammation and neurological damage.
The fact that the severity of the symptoms can vary greatly makes diagnosis of this rare disease difficult. Whereas women who carry and inherit the gene mutation are only mildly affected and often only develop (usually mild) symptoms between the ages of 50 and 60, the men always develop the disease, and they do so earlier and with more severe symptoms.
Cerebral adrenoleukodystrophy (CALD)
CALD is a rare, hereditary metabolic disorder that damages the brain and spinal cord. The blood-brain barrier becomes permeable, triggering inflammatory processes in the brain. Due to a gene mutation on the X chromosome, men always develop the disease. CALD usually begins with boys between the ages of 6 and 12, causing severe motor and neurological damage. The prognosis for those affected depends on early diagnosis and treatment.
Brain-damaging type in boys under the age of 12
Almost half of the men develop a slowly progressing adrenomyeloneuropathy (AMN) between the ages of 20 and 30, which results in motor problems and walking disorders. The brain-damaging form of ALD, cerebral ALD (CALD), on the other hand, has highly dramatic consequences.
It usually begins between the ages of 6 and 12 and is often unspectacular at first. The boys suddenly appear very clumsy – bumping into furniture, stumbling – and inattentive. They find it difficult to read, write and understand written texts. Some become aggressive or hyperactive. The literature reports a number of other symptoms such as swallowing disorders, strabismus, loss of hearing and vision. Misdiagnoses such as ADHD or “mental health problems” are relatively common.
The symptoms are signs that the protective myelin layer around the nerve fibers is dissolving. This important blood-brain barrier becomes permeable, activated immune cells can then penetrate the brain and trigger a self-reinforcing loop of inflammatory processes and myelin degradation.
If the aggressive form of ALD is not recognized and treated immediately – or as early as possible – the condition of the boys deteriorates rapidly. Within a few months, they are immobile or comatose and usually die within one to three years.
Is a virus a potential trigger of cerebral ALD?
There is no clarity yet about what exactly triggers the dramatic progression. “We know of identical twins, where only one of them has developed CALD,” says Weinhofer, who has been researching X-ALD for many years. “This shows that additional triggers are needed besides the gene mutation.” As findings from multiple sclerosis (MS) suggest, a virus could be the culprit (NB: MS is the most common demyelinating disease). Given that the time window in which the boys develop symptoms is so limited, hormonal factors would also be plausible.
There is no cure for the disease. If detected in time, further deterioration can only be halted with a bone marrow transplant. If no suitable donor is found, (very expensive) gene therapy is available in some countries.
It should also be mentioned that X-ALD became famous through the movie “Lorenzo's Oil”. The oil patented by Lorenzo Odone's parents can normalize the levels of super-long-chain fatty acids in the body of patients, but cannot prevent the onset of the disease. Hence, the clinical benefit is controversial. International guidelines do not recommend routine use.
“Time is of the essence after the onset of cerebral ALD,” emphasizes Weinhofer. Time, and an easy-to-use, reliable early detection method. At the moment, diagnosis is still complicated and stressful.
Difficult and nerve-wracking diagnosis
Some countries, including the USA and the Netherlands, offer a screening for newborns. While in favor of such screenings in principle, geneticist Weinhofer also mentions the disadvantages: all those diagnosed with X-ALD will develop the disease sooner or later (there is no cure) – but “only” half of them will develop the cerebral form. For families, the diagnosis means years of anxiety and uncertainty. From the age of six, the diagnosed boys are subjected to a magnetic resonance imaging (MRI) scan every six months to show whether the blood-brain barrier is already permeable and the brain’s white matter is inflamed.
These frequent MRI scans are also problematic: “Young children often have to be sedated for this,” says Weinhofer. “They are administered gadolinium, a contrast agent that can accumulate in the brain – with unknown long-term consequences.” In addition, the damage sometimes develops atypically and the MRI scan is more difficult to assess. “In that case the window of opportunity in which a transplant makes sense may already be closing.”
The location and extent of the lesions in the brain are assessed by means of the so-called Loes score of up to 34 points. Transplants are only performed up to a score of 9, after which the destruction is too advanced. Families may find this difficult to comprehend, as the children often seem to be still in relatively good health at this stage.
Clinical project in search of early markers
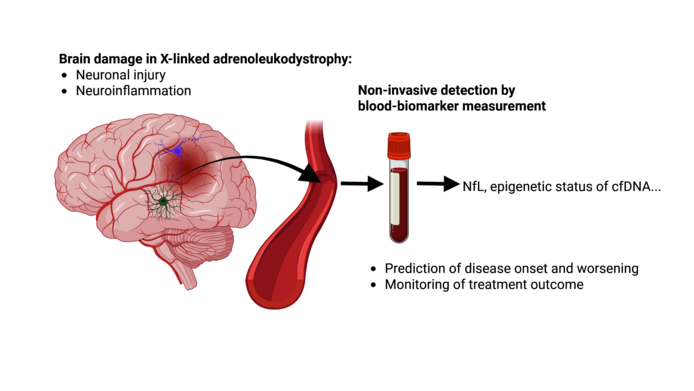
In the FWF-funded clinical project “Liquid biopsy for peripheral disease monitoring in X-ALD“ (2020–2025), Weinhofer seeks to develop a less invasive blood test. At the moment, she sees two promising candidates:
One is a protein called neurofilament light chain (NfL), which is released in all forms of neuronal damage (such as MS or Alzheimer's disease). In X-ALD, it rises to extremely high levels as soon as the nerves become inflamed. “We were able to show for the first time that NfL is already greatly elevated when only small lesions are visible on the MRI, but the blood-brain barrier is still intact,” notes Weinhofer. Boys in whom the inflammation is incipient have a significantly higher NfL value than X-ALD patients who do not develop brain inflammation. “NfL could therefore be an early marker for the onset of cerebral ALD.” Weinhofer also wants to find out at what NfL value a spinal cord transplant is still useful. “As soon as we have enough data here, parents could make more informed decisions – and we would have a complement to the less accurate Loes score.”
App designed to determine critical inflammation values
Her goal is an app for monitoring children with X-ALD. “Specialists could enter the children's NfL values and they would be compared with the data from a control group of healthy children. This would give us a score that shows the onset of neuroinflammation.”
For X-ALD research that would mean valuable data so that the time of onset of cerebral ALD could be narrowed down more precisely via NfL. “Although we receive blood samples from X-ALD centers all over the world, the overall number of cases is so low that it takes forever to get reliable values. This is a typical problem of conducting research into rare diseases.”
DNA traces of dead cells in the blood
A second interesting marker that Weinhofer's lab is researching is DNA traces of dead cells that are released into the blood. “Each cell type has a very specific pattern. If I find these traces in the blood, it tells me that this cell type is dying – in the case of cerebral ALD that would mean immune cells in the brain.” As cells are constantly dying in every organism, it is “a search for a needle in a haystack”. Weinhofer is working with a highly specialized bioinformatics laboratory in Israel, which tracks down cell-free DNA (cfDNA). “This could enable us to detect the onset of CALD much earlier than via NfL,” notes Weinhofer, who is happy about the initial positive findings. “In a follow-up project, I would like to confirm these results with larger numbers of patients. If that is successful, we could put together a blood test kit for early detection, which would really be a huge relief for those affected, but also for the clinics treating them.
About the researcher
Isabelle Weinhofer, a graduate of biology and genetics, is an assistant professor at the Center for Brain Research at the Medical University of Vienna, where she is in charge of a working group in the Department of Pathobiology of the Nervous System. Her research focuses on changes in lipid metabolism that lead to neuroinflammation and the effects of such inflammation on different brain cell types. Her previous work on the role of macrophages has contributed significantly to the understanding of stem cell transplantation as a life-saving intervention in X-ALD. From 2008 to 2010, she did postdoctoral research at ETH Zurich with an Erwin Schrödinger Fellowship funded by the Austrian Science Fund FWF. An FWF-ESPRIT position (formerly Hertha-Firnberg) enabled her to return to the Center for Brain Research in Vienna.
About the project
The early detection and assessment of the progression of the rare neurodegenerative disease X-linked adrenoleukodystrophy (X-ALD) is complex and very stressful for those affected and their families. The clinical research project “Liquid biopsy for peripheral disease monitoring in X-ALD” identifies and tests blood biomarkers that could significantly improve diagnosis. The project is awarded EUR 383,000 in funding from the FWF and will run until the end of September 2025; a follow-up project is currently being submitted.
Publications
Nine publications have appeared as part of the project and two more have been submitted. Most importantly:
Isabelle Weinhofer et al.: Neurofilament light chain as a potential biomarker for monitoring neurodegeneration in X-linked adrenoleukodystrophy, in: Nature Communications 2021
Isabelle Weinhofer et al.: Biomarker-based risk prediction for the onset of neuroinflammation in X-linked adrenoleukodystrophy, in: EBioMedicine 2023