Understanding Alzheimer's: protein migration in the brain

Alzheimer's disease (AD) is the most common form of neurodegenerative diseases. In the course of time, the death of nerve cells – more precisely, of the neurotransmitter acetylcholine – results in memory loss. Almost all cases of Alzheimer's (99 percent) are “acquired,” meaning they have no hereditary history. Growing older increases the likelihood of developing the disease, roughly from the age of 60, which makes aging the biggest risk factor.
Alzheimer's disease is typically characterized by changes in certain proteins in the brain: β-amyloid and tau protein. Some forms of β-amyloid (especially β-amyloid 42) tend to clump together and form the dreaded plaques outside the nerve cells which alter the signal transmission of the synapses and can trigger inflammation. New therapeutic agents (vaccinations) can reduce β-amyloid and slow cognitive decline, but they can also have serious side effects.
The second protein, tau, is a structural protein. It stabilizes certain components inside nerve cells. In Alzheimer's disease, tau mutates: it hyperphosphorylates – which means it accumulates too many phosphate groups – and forms clumps inside the cell.
Better understanding Alzheimer's
One of the big questions in dementia research is how the deposits (plaques) form and how they spread. Christian Humpel from the Medical University of Innsbruck has successfully developed a method for observing the spread of the proteins.
Testing the spreading hypothesis
There are a number of hypotheses concerning the reason for these deposits and what promotes their spreading in the brain. Alzheimer expert Christian Humpel from the Medical University of Innsbruck has dedicated his research career to these questions. In the project “Spreading of Alzheimer's pathology in organotypic brain slices”, which ran until the end of 2024, Humpel employed a method he has refined over almost three decades to examine the spread of the proteins in more detail: brain slices from mice.
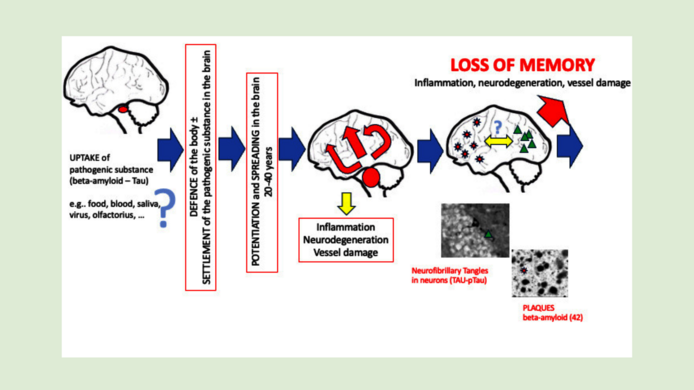
“We know that the first protein changes can occur from the age of 30, decades before the first symptoms appear,” explains Humpel. The spreading or propagation theory states that the defect spreads exponentially from one area of the brain to neighboring regions. At first, this happens very slowly – and in fact, those affected show no symptoms for many decades. However, from around the age of 60 or 70, the spread accelerates so rapidly that the entire brain can be affected. “We can picture it like an avalanche,” notes Humpel. The memory loss typical of Alzheimer's disease will set in only when a large proportion of the nerve cells has died and massive clumps have accumulated in the brain.
Mutated proteins migrate to the brain
A theory in Alzheimer's research that is now considered probable but has not yet been conclusively verified says that the diseased proteins behave similarly to prions, which are the triggers of Creutzfeldt-Jakob disease, mad cow disease, and Kuru disease, among others. Kuru has been found in Papua New Guinea among peoples who ritually consume the brains of the deceased.
“According to the prion hypothesis, a mutated, pathogenic protein somehow enters the brain – perhaps via food – and alters a healthy protein in such a way as to render it pathogenic,” says Humpel. A cascade reaction ensues in which other healthy proteins are infected. Although immune cells in the brain – microglia – ceaselessly work to correct errors, they get tired with age.
State award for brain slices instead of animal experiments
Humpel used three-dimensional “organotypic brain slices” to explore whether the spread occurs along specific pathways – a method for which he received the 2000 State Award for Alternative Methods to Animal Testing. In order to obtain brain slices, researchers remove the brains of eight- to ten-day-old mice (“organ removal”) and cut them into 150-micrometer-thick slices using a vibratome, “not unlike a sausage slicer.” The brains come from healthy animals and from genetically modified mice bred specifically for Alzheimer's research, which develop plaques themselves.
Organotypic means that the original cell architecture and the interaction of the cells remain intact to work in a similar way to the living organ: with nerve cells, glia cells, and blood vessels. “This offers the advantage that we not only study individual cell types, but can also observe their interaction within an existing brain architecture.”
In a next step, two brain slices are placed on special membranes, arranged side by side, and cultivated (typically for about two to four weeks). The researcher applies pathologically altered β-amyloid or tau protein to the upper of the two slices. The proteins are introduced into a tiny collagen bead, which slowly releases the protein as the collagen dissolves. The researcher then observes whether and how the protein spreads into the second brain slice. In a third series of experiments, Humpel also tested α-synuclein and was able to demonstrate that the spreading hypothesis may also be pertinent for the spread of Parkinson's disease.
Migrating proteins and defense reactions
The experiments support the spreading hypothesis: β-amyloid spreads into neighboring tissue, and tau migrates along nerve cell connections from the upper to the lower slice. Further research is now needed to clarify whether findings obtained from young mouse brains are applicable to human brains. Further experiments might involve tests on brain slices from adult mice or from deceased humans.
“What we also saw in the experiments is that the microglia defend themselves against the damaged protein: they gobble it up,” Humpel continues. On the other hand, the avalanche-like spread of proteins in the brains of Alzheimer mice that had already formed plaques was significantly faster. “So there are likely to be individual preconditions that promote Alzheimer's – or stave it off.”
It is now known, for instance, that an active lifestyle is associated with a lower risk of Alzheimer's dementia: plenty of exercise and a healthy diet could contribute to prevention, as could “brain exercise” through various activities, lifelong learning, and social contacts.
“I always tell my students that we can create the conditions that prevent disease ourselves: with everything that keeps the blood vessels in the brain in good shape.” But Humpel would also like to see more basic research. “Society is aging, and the number of Alzheimer patients will rise dramatically in the coming years,” he notes. “If we finally were to understand the disease in all its details, we might be able to prevent it much earlier. That would help millions of people and save enormous costs.”
About the researcher
Christian Humpel studied biology in Innsbruck, where he obtained his habilitation in 1996 after a Schrödinger Fellowship at the Karolinska Institute in Sweden. In 1998, the neurobiologist was appointed head of the Laboratory for Experimental Psychiatry at the University Clinic for Psychiatry at the Medical University of Innsbruck, where he established a research focus on Alzheimer's disease. He was awarded a professorship in 2019.
A second focus of Christian Humpel's research is the development of new biomarkers in cerebrospinal fluid, blood, and saliva for the diagnosis of Alzheimer's dementia. He received the Otto Löwi Prize from the Austrian Neuroscience Society for his research and recently received the Tuba Prize for his life's work. The project “Spreading of Alzheimer's pathology in organotypic brain slices” ran from 2020 to the end of 2024 and received EUR 393,000 in funding from the Austrian Science Fund (FWF).
The spreading hypothesis
Severe neurodegenerative Alzheimer's dementia causes protein plaques to form in the brain and nerve cells. It is still unclear why and how these deposits develop. The spreading hypothesis suggests that the proteins mutate and spread from one region of the brain to another. The aim of the FWF project was to study this hypothesis using three-dimensional organotypic mouse brain slices. The researcher succeeded in confirming the spreading.
Publications
Out of the 16 publications relevant to the project, here are the three most important ones:
Spreading of Aggregated α-Synuclein in Sagittal Organotypic Mouse Brain Slices, in: Biomolecules 2022
Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures, in: Biomolecules 2022
Spreading of Beta-Amyloid in Organotypic Mouse Brain Slices and Microglial Elimination and Effects on Cholinergic Neurons, in: Biomolecules 2021





