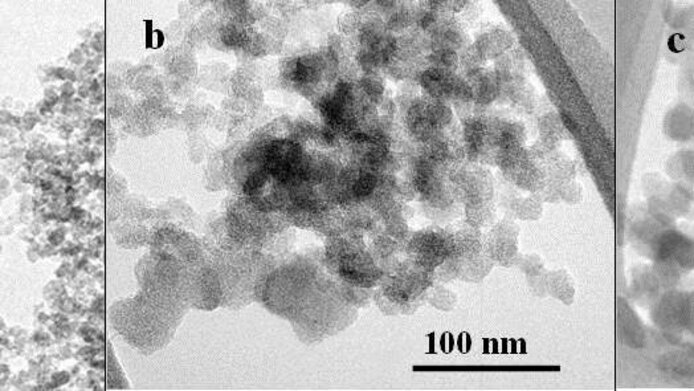
The formation of ice clouds

Cirrus clouds influence the Earth’s radiation balance and, thus, the climate. Depending on their distribution in space and time, these high ice clouds may either have a warming or cooling effect on the Earth's surface. For this reason, it is all the more important to understand exactly how water droplets in clouds turn into ice crystals. The Intergovernmental Panel on Climate Change (IPCC) considers the interaction of suspended particles and cloud droplets as one of the fields in which scientific knowledge is lacking. Led by the atmospheric chemist Hinrich Grothe, a team of researchers at TU Wien (Vienna University of Technology) has joined forces with Regina Hitzenberger (University of Vienna) and Thomas Lörting (University of Innsbruck) to explore the process of “ice nucleation” as it takes place in clouds. With the support of the Austrian Science Fund FWF, the researchers have investigated the composition of carbonaceous particles right down to the level of individual building blocks and studied the interplay between molecular structure and chemical composition on their surfaces as a basis for ice formation.
Catalysts during ice formation
Ice clouds form in the middle and upper troposphere, between five and fifteen kilometres above the Earth's surface. In pure water, the transition from water droplets to ice crystals occurs only at very low temperatures (around minus 38 degree Celsius). Small particles, so-called ice nucleation particles, which are transported from the Earth's surface to the upper layers of the troposphere or emitted directly from aircraft engines, can act as “catalysts of ice formation”. Under these circumstances, the phase change from liquid water to ice at or around the ice nucleation particles takes place at higher temperatures. In the troposphere, mineral dust and biological material (pollen, fungal spores, macromolecules) as well as soot particles from combustion processes often act as such ice nuclei.
“We wondered what it is that makes an ice nucleation particle a particularly good ‘catalyst’. Since soot is abundant in the troposphere, we have compared different types of soot particles with other potent ice nuclei and analysed their possible interaction with water down to the molecular structure,” explains the principal investigator Hinrich Grothe from TU Wien. The analysis has provided a Solomonic answer to the question as to whether it is the molecular structure or the surface composition, i.e., the physics or the chemistry, of the ice nucleation particles that has a greater significance for the potent formation of ice: they are both important. In case of soot its ability to act as ice nucleator even changes with time, as it becomes more and more oxidized at its surface in our atmosphere.
Soot evolves over time
Anyone who has ever tried to use pure water to wash soot off a surface knows that these two substances do not exactly mix well. However, biological materials as well as soot particles change over the course of weeks spent in the atmosphere. In the test series, the team was able to show that chemical compounds attach to them or wash out, which modifies the surface and structure of the particles. This can enhance or weaken their ice-forming potency. Moreover, soot particles are not necessarily identical, even if they come from the very same combustion engine. In the laboratory, the researchers specifically produced and modified a variety of soot particles and tested and characterised their ice-forming potential. As a simplified model, they also tested graphene, which can be regarded as a basic building block of soot. The team compared the soot particles with biological material from the bark and leaves of birches, which are natural freeze survivalists, as well as Pseudomonas syringae, a bacterium used in producing artificial snow. The results reveal that soot particles, while not exactly super-active in ice nucleation, are important “catalysts” for climate-relevant ice cloud formation because of their wide distribution, length of stay in the atmosphere and variability through chemical reactions. Hence, they represent a relevant factor for calculating radiation balances and for the spatial and temporal distribution of ice clouds.
Personal details Hinrich Grothe from the Institute of Materials Chemistry at TU Wien acquired his professorial qualification in physical chemistry. He studied in Dresden and Hanover and obtained his doctorate in low-temperature spectroscopy. Grothe has been studying the formation of ice clouds for many years, and he heads the Atmospheric Chemistry and Aerosols Section of the European Geosciences Union (EGU).
Publications