Patented turbo for immunotherapies against cancer

More and more cancer therapies are relying on the effect of immune cells. Oliver Spadiut, Professor of Bioprocess Engineering at the Vienna University of Technology (TU Wien), is conducting research into how such cells can be multiplied in the laboratory and biotechnologically modified in order to kill cancer cells as effectively as possible. In the context of his FWF-funded project “Digital twin-assisted process design for NK-cell therapies”, Spadiut has now succeeded in developing and patenting novel cultivation strategies for certain immune cells. This will significantly reduce the production time for cell therapies – and perhaps also shorten the waiting time for patients.
No longer weeks, but days
“Our discoveries reduce the cultivation period for obtaining a clinically relevant dose from around four weeks to just eight days. This is significant progress in therapeutic use,” emphasizes Spadiut. The underlying idea of such cell therapies is to multiply immune cells from patients or from established cell lines in a bioreactor and subsequently administer them to the patients.
With his team, Spadiut and Valentin von Werz are working on natural killer cells (NK cells), a subgroup of white blood cells that can kill tumor cells in a targeted manner. Researchers are increasingly focusing on NK cells as a supplement to the T-cell therapies already commercially available (known as CAR-T cell therapy). “Even slightly more potent than the T cell, NK cells are currently being investigated in advanced clinical trials,” reports Spadiut.
Many cancer treatments rely on immunotherapies. In the international WEAVE project, co-funded by the Austrian Science Fund FWF, researchers have succeeded in speeding up the proliferation of patients’ immune cells. Not only can patients be treated more quickly and with more cells, but the cells also become more effective.
Patented turbo for killer cells
“For therapeutic use, you have to grow the NK cells quickly in large numbers and also ensure that they remain highly cytotoxic – i.e. that they kill off cells. And therein precisely lies the challenge. You often lose weeks in cultivation, and in the end the cells no longer have an effect when you administer them to the patient,” explains Spadiut. The project has already resulted in two patent applications and the research group is currently working on four scientific publications.
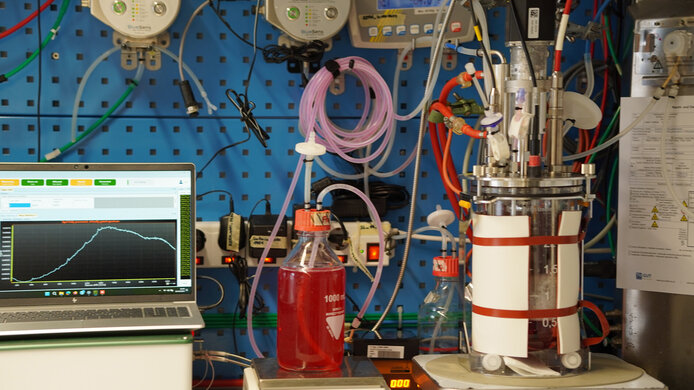
The challenge in cultivating these special cells is that NK cells lose their cytotoxicity at low pH values. “Something that hadn’t been understood until now: it is not the pH value itself that makes the difference, but the lactate produced,” notes Spadiut. Lactate, the salt of lactic acid, is produced during energy production in cells. “We discovered that NK cells have a lactate threshold value – once this threshold is exceeded, they lose their cytotoxicity. We then developed a novel cultivation strategy, a lactate auxostat that measures the lactate concentration in the bioreactor and automatically adjusts the culture.”
Plenty of potential for clinical practice
For the second patent application, Spadiut and von Werz investigated the relationship between lactate and cytotoxicity. “NK cells have many surface markers that interact in complex manners. One of them is called FasL (Fas ligand). When this ligand binds to its respective receptor on a cancer cell, it triggers programmed cell death there,” explains Spadiut.
The data collected in controlled cell culture experiments were then modeled as a digital twin. The researchers learned that if the lactate concentration exceeds a certain threshold value, this death ligand, which triggers cell death, does not appear. Many solid tumors exploit this mechanism by protecting themselves with a lactate envelope.
“However, if we add the death ligand externally, the NK cells suddenly become active again,” reports Spadiut. For this reason, the second patent application aims to create particularly effective NK cell lines that produce more of this death ligand. “We hope that this will also help us fight solid, encapsulated tumors in the future – that would be a novel feat, because cell therapies so far have mainly been used for non-solid cancers, such as blood cancer,” says Spadiut.
From digital model to real therapy
It was the objective of this FWF-funded project to map the physiological network of the cell culture in a digital twin. Spadiut now intends to derive parameters that will permit individually optimized cultivation conditions for each patient in the future.
“Our research at TU Wien is very application-oriented. I think it is precisely because we are not medical doctors that we were able to find these new approaches – because we want to understand every detail of the biotechnological processes,” explains Spadiut. His vision is to transfer the cultivation processes into a scalable, controllable system while also obtaining new insights into NK cells. In this way, basic research and clinical application are to be brought even closer together in future in order to fully exploit the medical potential of this cell therapy.
The researcher
Oliver Spadiut is a biotechnologist and heads the research groups “Integrated Bioprocess Development” and “Bioprocess Technology” at TU Wien. He was appointed Head of the Bioprocess Engineering Research Unit in 2022. In his research, Spadiut investigates and develops biotechnological production processes, particularly for biopharmaceuticals. The international WEAVE project “Digital twin-supported process design for NK cell therapies” (2022-2025) has been awarded roughly EUR 230,000 in funding by the Austrian Science Fund FWF.