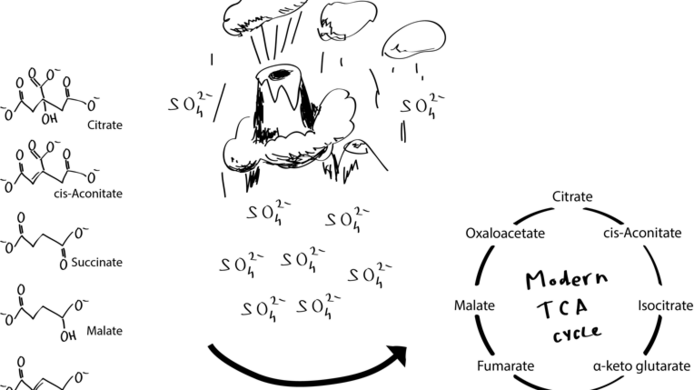
The Archean ocean as an ideal breeding ground for life

The origin of life is perhaps the greatest mystery of science. It is still not adequately understood how something so complex could evolve from inanimate nature. The biochemist Markus Keller from the Medical University of Innsbruck has now made an important contribution to our understanding of how life developed on Earth. An Erwin-Schrödinger Fellowship from the FWF enabled Keller to do research abroad. In the course of his work he explored how some very old and complex processes of cellular metabolism developed. – Processes that are almost four billion years old and are also found in the human organism. “The crux here is how metabolism started in the first place”, says Keller in the interview with scilog. “In some places on our planet there are very old sediments showing that life began more than 3.7 billion years ago. From these sediments we are unable, however, to conclude in exactly what form life existed and what its characteristics were. We just know that there must have been some kind of metabolic activity”, notes Keller. Some metabolic pathways are identical in nearly all living organisms on the planet. One example is glycolysis, the processing of sugar. “Plants, bacteria and other living organisms use glucose in the same way we ourselves do. We may assume that the processes were the same in life-forms existing at very early stages of evolution. The question is this: how could these life-forms interconvert the intermediate products of glycolysis?”
The mystery of missing enzymes
Cellular metabolism is a complicated system that depends on a number of enzymes. – These special proteins serve as catalysts, and some processes would not be possible without them. If an enzyme is missing, the entire cycle does not work. As Keller explains, it's a chicken-or-egg problem: what came first? The enzymes, which are metabolic products themselves? Or metabolism, which does not function without enzymes? Only a few years ago, the idea that several of these metabolic mechanisms might have functioned without enzymes, simply because of the prevailing environmental conditions, was disparaged as “magical thinking”. But it is precisely these processes whose existence Keller was able to demonstrate.
Importance of iron in the Archean ocean
His first papers dealt with glycolysis and what is called the “pentose-phosphate pathway”. “At the time when life must have begun, the Archean ocean was relatively warm and contained a great deal of iron in a dissolved state”, explains Keller. Under normal circumstances, iron is not water soluble in its oxidised form, i.e. rust. About four billion years ago there was, however, hardly any pure oxygen in the atmosphere or in the ocean which would have supported iron oxidation. Therefore, there existed large quantities of iron (II), or ferrous iron, which is easily dissolved in water. “We simulated the conditions prevailing in the Archean ocean and looked at how, for instance, fructose-6-phosphate, an intermediate product of cellular metabolism, would react in this environment. One of the things we found was that it converts to glucose-6-phosphate, precisely the same sequence of reaction and reaction pathways as in the living cell. In the first publications we showed that this occurs in a surprisingly efficient manner with very few side reactions. It results in exactly the right molecules.”
Metabolic processes first, enzymes next
For this reason, the Archean ocean was an absolutely ideal environment for these very old metabolic reactions. And here lies the solution to this particular chicken-or-egg problem: chemical metabolic pathways were there first, and the enzymes developed later. Keller was able only recently to demonstrate a similar situation for the “citric acid cycle” (CAC), another important part of cellular metabolism. Its individual reactions can also run in the absence of enzymes. Analogous to modern cells, where glycolysis and the CAC, which is located in the cell mitochondria, run separately in different milieus, their non-enzymatic counterparts also need different chemical milieus in order to run effectively. In this way, the researcher showed that the observations made in relation to glycolysis also applied to other important metabolic pathways.
New methods trigger ideas
Keller was able to make these observations by using mass spectrometry methods he developed during his Schrödinger Fellowship at the University of Cambridge. Mass spectrometry is an extremely sensitive method of measuring involving the breaking down of substances into their individual molecules or atoms in order to determine their mass. Keller originally examined how the components of yeast cells could be analysed by means of mass spectrometry, since it was not only highly precise but also promised additional advantages over other methods. Yeast is one of the most important model organisms of biology, and Keller’s work was basic research with the aim of developing methodology for other types of research. He developed the idea of looking at the evolutionary origin of cellular metabolism together with the microbiologist Markus Ralser, head of the Cambridge research group of which Keller was a member. They also asked Alexandra Turchyn, an expert on Archean oceans, to join them and published the first paper on this issue.
Important side-line observations
“Actually I never planned for my research to go in this direction”, says Keller. “The initial study done on yeast metabolism is now also awaiting publication. But it was important that I had the freedom to look into these things. At first it was just a side-line.” Keller emphasises that some of these effects have probably been measured in other studies as secondary effects but were not reported in detail. “These reactions still occur in cells today”, observes Keller. He encourages groups that are active in this field to take a closer look at what they may misinterpret as being measuring errors.
Personal details Markus Keller is a biochemist at the Division of Human Genetics at the Medical University of Innsbruck. His research focuses on the control, properties and evolution of cellular metabolism, particularly reaction mechanisms in the absence of enzymes, as well as energy and membrane lipid metabolism in modern organisms. Between 2012 and 2015 a Schrödinger Fellowship from the FWF took him to the University of Cambridge.
Publications