Medication that hits the mark
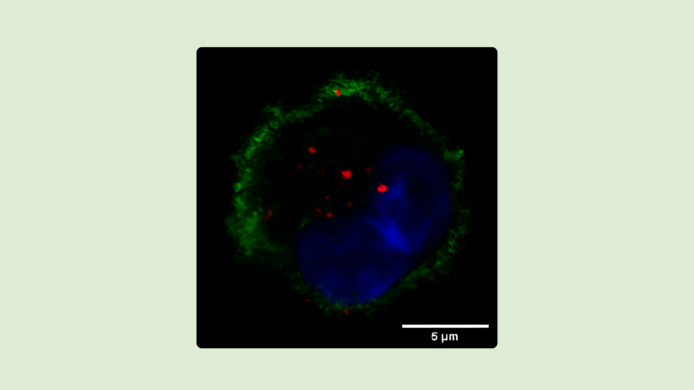
Even the best medication is ineffective if it does not reach the right spot. One way to help it arrive on target is to use receptors that occur only in certain cell types. “We use a receptor named Langerin, which is exceptionally suited to absorbing and processing substances and manipulating cells in a targeted manner. This receptor is specifically found in Langerhans cells, a type of immune cell in the skin,” explains Christoph Rademacher, Professor of Molecular Drug Targeting at the University of Vienna.
His team has succeeded in developing highly specific and efficient systems that dock onto the Langerin receptor, thereby opening up potential therapeutic approaches for autoimmune diseases, allergies, infectious diseases or even vaccines against cancer. The findings originate from Rademacher's earlier work at the Max Planck Institute in Potsdam before his research took him to Austria. Right now, his team is working on further developments of the systems, also in the context of the “Ligand-based delivery vehicles for murine Langerin” research project which is funded by the Austrian Science Fund FWF. Initial findings are currently being processed for publication.
New pathways for active ingredients
Rademacher considers research into targeted drug delivery systems a key aspect of modern drug development, both for drugs based on the CRISPR/Cas9 gene scissors and those that take effect at the RNA level. “The crucial question being: how do I get the active ingredient into a specific cell without affecting other cells? That's what we're working on,” notes Rademacher.
Rademacher's research is based on the fact that Langerin is naturally activated by certain sugars. In earlier work, he developed a compound with an optimized sugar-like structure, a glycomimetic, which becomes effective specifically in the Langerhans cell. “In my current research, I am investigating which changes occur in the pathway from receptor activation to cell uptake and effect depending on which binding substance, i.e. which ligands, we use,” explains Rademacher.
Research into targeted transport systems is a key aspect of modern drug development. Austrian researchers such as Christoph Rademacher play a key role in this development. The biotechnologist is cofounder of Cutanos GmbH in order to build the bridge to application in clinical studies.
Basic research increases effectiveness
The development of a glycomimetic is a complex undertaking. First, the researchers had to decipher in detail the molecular structure of the Langerin receptor in order to identify specific binding sites and potential ligands. For this purpose, Rademacher's team cooperated with the Medical University of Innsbruck and a group at the Leibniz Research Institute for Molecular Pharmacology in Germany. “The commercial development of targeted delivery systems often leaves no time for this molecular-level research, but it provides crucial insights,” emphasizes Rademacher.
Analyzing promising ligands in cell biology and biophysical experiments, the Vienna research group was able to make important progress: “We have developed novel ligands that do not necessarily have to be glycomimetics, but can specifically influence the uptake of such a receptor system – for instance in terms of uptake rate or receptor recycling in the cell,” says Rademacher. His many years of experience with Langerhans cells are certainly of benefit in this context.
Sentinels of the immune system
In the development of new drugs, the role of the immune system is becoming increasingly important, particularly if it is to be targeted by immunotherapies. Langerhans cells are special immune cells in the uppermost skin layer. Rademacher is convinced that this cell type has great potential, although there is still little research on it. Langerhans cells have two main tasks: first of all, they take up pathogens when they enter the body. This makes them the first cells to be infected when HIV is transmitted. Transport systems that specifically deliver vaccines to these cells could help the immune system to build up effective protection.
On the other hand, Langerhans cells act as sentinels of the immune system in a state of rest. On their surface, they present structures of foreign substances, so-called antigens, and they regulate the immune response. This characteristic is harnessed in diseases in which the body's own structures are erroneously identified as foreign and the immune response goes into overdrive. “When Langerhans cells present antigens in a resting state, they trigger tolerance in the body’s T cells – they constrain the immune response against these structures,” explains Rademacher. “Meaning that if we can sneak a structure into the Langerhans cells without activating them, we could use this for the treatment of autoimmune diseases and allergies.”
Still at the beginning
Many targeted delivery systems are still in the research phase, but initial successes have already been achieved. “The first active ingredients that specifically target liver cells via sugar compounds have been on the market since 2019,” says Rademacher. The underlying technologies employ a variety of biological tricks: in addition to glycomimetics, some rely on lipid nanoparticles that contain, e.g., mRNA vaccines, while others couple RNA-based active ingredients directly to small chemical compounds, so-called small molecules, which dock specifically to cells.
Drugs that target the Langerin receptor are also being tested for applications. Rademacher is one of those involved in such tests, as he, apart from his academic research, is also co-founder of the Vienna-based spin-off Cutanos. “Cutanos has already achieved promising results with this approach in studies with animal models. We are now actively looking for further investors to advance the clinical trials,” notes Rademacher.
Without basic research, such applications would not be possible. Conversely, the prospect also offers great advantages for academic research, says Rademacher: “In my lab, we cover a broad field of research – from structural biology and small molecule synthesis to cellular immunology in animal models. It is a huge motivation for my team to see that our research doesn't just stay in the lab, but potentially contributes to helping patients in the future. We work with our sights on the application.”
Personal details
Christoph Rademacher has held a professorship at the University of Vienna and the Max F. Perutz Laboratories since 2020. He studied Molecular Biotechnology and Molecular Life Science and worked at the Scripps Research Institute in the USA as well as at the Max Planck Institute of Colloids and Interfaces in Potsdam. In his research, Rademacher develops glycomimetics and other technologies for cell-specific targeting. He is co-founder of Cutanos GmbH. The project “Ligand-based delivery vehicles for murine Langerin“ (2021-2025) receives roughly EUR 167,000 in funding from the Austrian Science Fund FWF.
Selected publications
Rahhal N., Rentzsch M., Seiser S. et al.: Targeted delivery of cytotoxic proteins via lipid-based nanoparticles to primary Langerhans cells, in: Nanoscale 2025
Lefèbre J., Falk T., Ning Y., Rademacher C.: Secondary Sites of the C-type Lectin-Like Fold, in: Chemistry 2024
Leusmann S., Ménová P., Shanin E., Titz A., Rademacher C.: Glycomimetics for the inhibition and modulation of lectins, in: Chemical Society Reviews 2023
Wamhoff E.-C., Schulze J., Bellmann L. et al.: A Specific, Glycomimetic Langerin Ligand for Human Langerhans Cell Targeting, in: ACS Central Science 2019