How to extract biofuel from yeast cells

Biologically produced fuel is one of the elements raising hopes for an energy shift in the future. Internal combustion engines running on climate-neutral diesel or petrol could support the phasing-out of fossil fuels in addition to e-mobility. At present, however, this "bio-fuel" has to be produced from high-quality raw materials such as rape or maize, and large areas of arable land are required to cultivate them. As a consequence, research groups from all over the world are intensively exploring alternatives. One of these would be to use yeast cells to produce fat from cellulose waste, which can then be converted into biodiesel. In a project funded by the Austrian Science Fund FWF, a research team led by the molecular biologist Klaus Natter from the University of Graz aimed at an increase in the production of fat in yeast cells through genetic modification.
Well-researched yeast
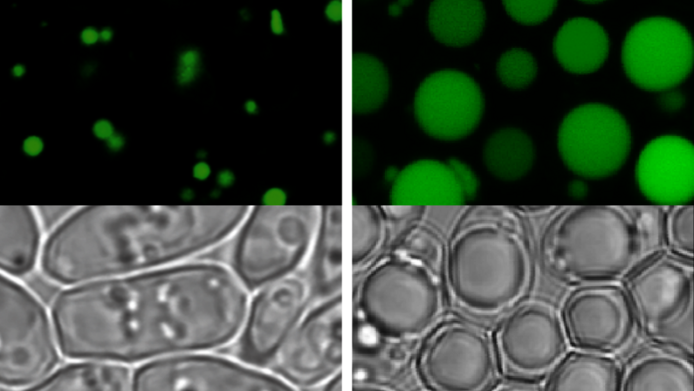
Natter and his group benefit from the fact that yeast cells have been very well researched. “The genomes of the yeasts we work with have been completely sequenced, and almost all metabolic processes occurring in the cell are known”, Natter relates in the interview with scilog. He is referring to what is otherwise known by the names of "baker's yeast" and “oleaginous yeasts”. “The former is the type of yeast we are most knowledgeable about. It is used in most foodstuffs, such as bread, beer and wine and, hence our interest, in biofuel production. Since the middle of last century it has been a popular yeast model in research. That's why we know much more about it than about any of the others”, Natter explains. Oleaginous – or fatty - yeasts, on the other hand, have only been used for a short time. “This type is of interest for us because of its high lipid content which gave it its name.” Fatty yeasts store up to 20 percent of their total weight in fat, baker's yeast between five and ten percent.
Up to 70 percent fat
Using computer simulations of cell metabolism, Natter's research group spent a year trying to create an exact model of the fatty yeast Yarrowia lipolytica that would depict all metabolic processes in the cell and to identify genes that could increase the fat content when modified. The modelling results were confirmed by subsequent experiments: the researchers were able to optimise both fatty yeast and baker's yeast in respect of their lipid yield. After genetic modification, the fatty yeast stored between 50 and 60 percent lipids. Surprisingly, even the lipid content of baker's yeast, naturally much lower in fat, was also increased several times over. “This shows that it is not entirely correct to divide them into fatty and non-fatty yeasts”, Natter explains. Baker's yeast, which is better understood by science, might even be the more promising candidate for lipid production. The researcher emphasises that the altered yeast cells are normally viable and merely grow at a slightly slower pace than their natural counterparts.
While Natter's group is satisfied with the results of this basic research project, there are still a number of hurdles before industrial implementation becomes viable. “In order to make the process sustainable, waste products would have to be used as the nutrient for these yeasts”, Natter notes. Cellulose decomposed by suitable enzymes would be a good candidate. “When harvesting a maize field, one would no longer use the cobs for biodiesel production, but the rest of the plant.” For the time being, however, there is no way of achieving this in a way that makes economic sense. According to Natter, there is only one serious competitor for yeast in terms of future biodiesel production: algae. Algae are attractive because they can convert sunlight and CO2 directly into fat, but they also require large areas and grow much more slowly than yeast. Yeast grows about ten times faster.
Yeasts that secrete diesel
Increasing the lipid yield of yeast is only one important milestone on the road to sustainable biodiesel production. Natter draws attention to two further research directions that will facilitate the process in the future. One important aspect is “cell disruption”, i.e. the question of how to get the fat out of the cell. “Extracting the fat droplets from the cell is a complex process”, Natter says. Making the cell itself excrete the fat it produced would constitute an important step towards biotechnological implementation. According to the researcher, there is currently no viable solution to this problem. There are alternative approaches aiming at making the yeast cells produce not only the fat, but the actual biodiesel, which could be secreted by the cells. While this can be done in principle, the yield is much lower than with fat storage in the cell. Further basic research should promote better understanding and optimisation of this process. It is hoped that yeast cells that feed on cellulose could in future produce biodiesel directly and excrete it independently. “Basic research into the production of fat from yeast has already reached a very advanced stage”, Natter confirms. “Now the time has come to put it into practice.”
Personal details Klaus Natter is an Associate Professor at the Institute of Molecular Biosciences, University of Graz. His research focuses on the regulation of lipid metabolism and application-oriented approaches to the development of yeast strains for the biotechnological production of lipids and lipid-like substances.
Publications