Hormonal balance between liver and brain

Reducing massive excess weight or preventing it from building up in the first place is the most important preventive measure against metabolic diseases such as type 2 diabetes or fatty liver. People are usually aware of the connection, but in the land of schnitzel and pastries many find it difficult to act accordingly: a permanent high-calorie diet coupled with too little exercise causes weight gain. Hormones, the central nervous system and the liver work closely together to control the body’s calorie intake.
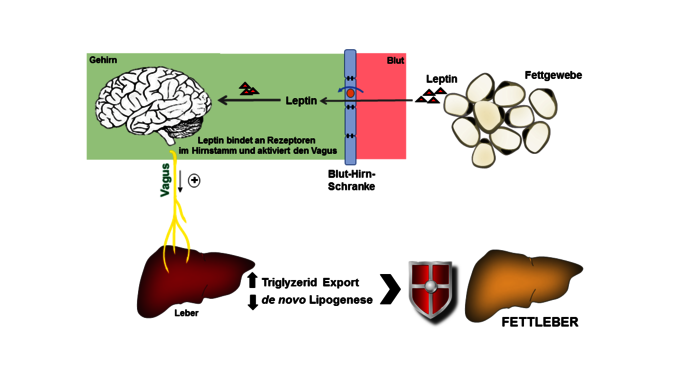
With funding from the Austrian Science Fund FWF, a team under principal investigator Thomas J. Scherer has conducted a series of experiments with (overfed) rats and mice. They have been able to show that the lipid metabolism is coordinated by the brain and regulated by the hormone leptin along a connection between the brain, the vagus nerve and the liver. After food intake, leptin released by the adipose tissue, its receptors in the brain, the autonomic vagus nerve – which connects the brain with many important organs and the intestine – and the liver do their work unbeknownst to us.
Fatty liver: number one among liver diseases
In a healthy organism, the liver converts blood sugar that is not immediately usable into fat, which the adipose tissue receives and stores as an energy reserve. When the adipose tissue is pathologically altered or reaches its capacity limits, fat is stored in the organs, including the liver itself. In Austria, non-alcoholic fatty liver (NAFLD) is the most common liver disease. It is associated with increased abdominal girth, elevated blood sugar, increased blood lipids, higher blood pressure and insulin resistance, also known as metabolic syndrome. As of now, there are no drugs to specifically treat fatty liver. Thomas Scherer, an internal medicine specialist at the Vienna General Hospital’s Division of Endocrinology and Metabolism emphasizes that in the foreseeable future non-alcoholic fatty liver (NAFLD) will replace hepatitis C as the leading pre-existing condition for liver transplants in western industrialized countries.
Mapping the entire lipid metabolism
The hormone leptin is produced in adipose tissue and penetrates the restrictive blood-brain barrier. It is even actively transferred through a transport channel and binds to receptors in the brain. In the brain, leptin conveys a sense of satiety and also provides information about the lipid mass and stimulates the export of triglycerides (dietary fats) from the liver to the adipose tissue. When the control circuit works normally, this fat removal signal is passed on to the liver via the vagus nerve of the autonomic nervous system. Pathologically obese people with fatty liver have an elevated leptin level in the blood, but the signal for fat removal probably does not reach the brain, because the circuitry of the brain-vagus-liver axis is disrupted.
“We are researching lipid metabolism in a holistic manner, not focusing on one single organ, and we also look at inter-organ communication. Once we have a better understanding of the signalling pathways, we may be able to derive therapeutic approaches. We have already obtained valuable data from experiments with model organisms. In the ongoing clinical study, which is also funded by the FWF, we are comparing liver activity and leptin regulation in healthy people, in liver transplant patients and in patients who, because of a rare disease, have no adipose tissue (lipodystrophy),” explains Scherer.
Leptin switch in the brain and connection to the liver
At the Medical University of Vienna (MUW), Thomas Scherer's team first investigated rodents. They injected leptin into various brain regions and then studied the animals’ liver fat metabolism. The current clinical study is aimed at investigating the effect of leptin and comparing healthy people with liver transplant patients whose organs do not have a grown nerve connection to their brain, as well as people who do not produce leptin due to illness and who present with extreme cases of fatty liver. A decisive factor for success is the intensive cooperation among departments at the Medical University in Vienna and with hospitals in Leipzig (Germany) and Pisa (Italy). State-of-the-art technology in the shape of the 7-Tesla Magnetom from Vienna is also used to investigate the physiological processes. With the help of this high-resolution magnetic resonance imaging and blood tests, the team can simultaneously examine liver function, fat content and triglyceride composition – with and without leptin administration.
Personal details
Thomas Scherer is an associate professor at the Medical University of Vienna. He has been researching at the Division of Endocrinology and Metabolism since 2011. Prior to that he held a postdoc position at the Icahn School of Medicine at Mount Sinai in New York City. His research group is focusing on the influence of the central nervous system on energy, glucose and lipid metabolism. He heads the Outpatient Clinic for Inherited Metabolic Diseases in Adults. Scherer is currently conducting a clinical study with FWF funds.
Publications
T. Hackl, C. Fürnsinn, C. M, Schuh et al.: Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis, in: Nature Communications Vol. 10, 2717, 2019
Scherer, C. Lindtner, J. O’Hare et al.: Insulin Regulates Hepatic Triglyceride Secretion and Lipid Content via Signaling in the Brain, in: Diabetes Jun; 65(6): 1511-1520, 2016
Scherer, P.Wolf, S. Smajis et al.: Chronic Intranasal Insulin Does Not Affect Hepatic Lipids but Lowers Circulating BCAAs in Healthy Male Subjects, in: The Journal of Clinical Endocrinology & Metabolism, Vol. 102, Issue 4, 2017