HIV vaccine: new tactics needed
As far back as in 1983, the virologists Françoise Barré-Sinoussi and Luc Montagnier discovered the human immunodeficiency virus type 1 (HIV-1). Now, 40 years later, researchers are still trying to come up with strategies against the pathogen that causes AIDS, the acquired immunodeficiency syndrome. “Viruses are masters of mutation, and this is particularly true of HIV. This virus has a very high mutation rate and is always one step ahead of our immune system,” says Doris Wilflingseder, Professor of Infection Biology at the Medical University of Innsbruck.
In her research, Wilflingseder focuses on the reactions of the human immune system to pathogens. In a project funded by the Austrian Science Fund FWF, her team characterized the signaling pathways that trigger a new infection with HIV. They discovered that the virus particles are enveloped by certain proteins that are crucial for the body’s response. The researchers now want to use the knowledge they have gained to develop a vaccine that mimics these signals in order to trigger a particularly strong immune response.
Hundreds of new infections every year
According to estimates by the Austrian AIDS Society, there are 9,000 people in Austria living with an HIV infection. Around 400 new infections are detected every year, but it may be assumed that one out of ten infected individuals has not yet been tested and is unaware of their infection.
HIV is transmitted via blood, semen, vaginal secretions and other infectious body fluids. Like all pathogens, the virus particles encounter an initial protective barrier (non-specific immune defense) upon entering the human body. This barrier suppresses the infection for the first 2 to 3 weeks until the specific immune response sets in, which involves HIV-specific immune cells (T cells) and antibodies. Wilflingseder discovered that during the acute phase the virus particles are enveloped in a coat of proteins. “Unlike 'naked' virus particles, HIV coated with acute-phase proteins triggers a stronger immune response,” Wilfingseder says, summarizing the results.
Personal details
Doris Wilflingseder is conducting research on the interactions between pathogens and their hosts at the Institute of Hygiene and Medical Microbiology at the Medical University of Innsbruck. In 2020, she was appointed professor of infection biology. In the context of her scientific work, Wilflingseder is also committed to research without animal testing, inter alia by founding the MUI animalFree Research Cluster. Her project “HIV-C escapes restriction, not sensing in DCs” is set to run until May 2024 and is being awarded EUR 407,000 in funding by the Austrian Science Fund FWF.
New strategy for a vaccine
The research results demonstrate that our immune system responds most strongly to an HIV infection in the acute phase. The effect becomes weaker when specific, non-inhibitory antibodies bind to the pathogen surface later on. “This is important because chronic activation of the immune response could lead to organ damage,” explains Wilflingseder.
In order to explore a new route for vaccine development, her team wants to recreate the reactions of the acute phase. “For this purpose we have conducted detailed research on the signaling pathways and mechanisms of the non-specific immune response,” reports Wilflingseder. “In collaboration with local, national and international experts, we are aiming to develop a vaccine that targets the same receptors as HIV coated with protein.” The intention is to have the vaccine trigger the more effective signaling pathway, which improves the ability of immune cells to take up foreign molecular structures and induce an immune response in the regional lymph nodes.
The complement system
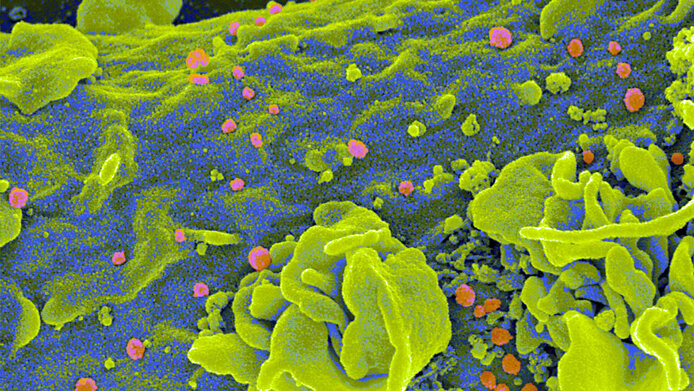
The mechanism that the researchers are exploiting is based on a known part of the non-specific immune defense that has hitherto been neglected in HIV: the complement system. This name refers to a group of soluble proteins that are activated by various pathogens and, in the best-case scenario, initiate their destruction, or otherwise keep the pathogen in check until the specific immune response becomes effective.
The proteins of the complement system are present, inter alia, in the blood and seminal fluid and form a coat around the HIV particle. When such a complement-coated virus encounters an immune cell, it is recognized by different receptors than naked HIV. “Previous research has tended to investigate the interactions of the 'naked' virus with isolated cell types. But the pathogen is not present in the body in this form. That's why for our experiments we have chosen to simulate the start of an infection as realistically as possible using cell cultures,” notes Wilflingseder.
What happens during the infection?
In the case of both HIV infection and a potential vaccination, the first immune cells to encounter the virus particle are the dendritic cells. “They are present in the tissue, and as 'sentinel cells' of the immune system react immediately when a pathogen enters the organism,” explains Wilflingseder. Firstly, they release soluble antiviral substances (interferons) which curtail the infection. Secondly, the dendritic cells “eat” and cut up the virus and migrate to the nearest lymph node, where they trigger the specific immune defense.
“If an HIV particle carries a complement coat, the dendritic cells will take it up and process it in a completely differently manner,” says Wilflingseder. Naked HIV particles are suppressed in the dendritic cell in the same way as the antibody-coated viruses at a later stage. This is why they only trigger a moderate immune response. In contrast, HIV enveloped by complement factors reaches the cell nucleus and infects the dendritic cells, which in turn triggers a strong immune response that can reduce the viral load in the acute phase.
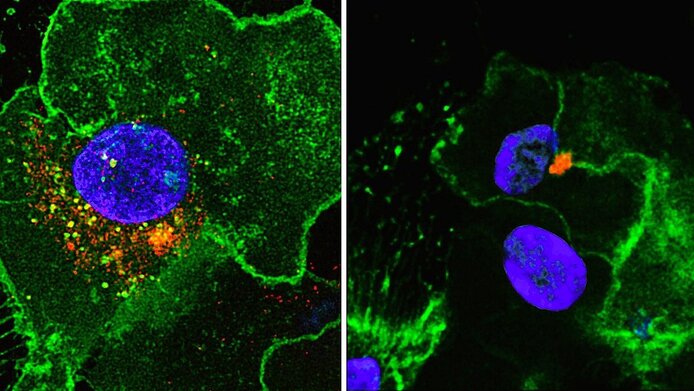
“The fact that the dendritic cells are infected is a two-edged sword,” Wilflingseder admits. “But we were able to show that a minimal productive infection of the cells helps the body to recognize the virus better.” The same effect is achieved by the less common HIV-2 subtype, which has been proven to cause AIDS more slowly than HIV-1.
A new approach required
“Vaccinations usually avoid activating the complement system in order to prevent excessive immune reactions. Our research findings suggest, however, that we need to rethink HIV vaccine development,” Wilflingseder points out.
This begs the question as to whether the HIV vaccine is now within reach after 40 years of research. “It goes without saying that a paradigm shift towards complement activation requires thorough investigation. It can only be achieved if the positive effects described in the cell culture are also observed in the living organism and if excessive immune effects are avoided – hence, it is difficult to predict how much time it may take,” says Wilflingseder. At any rate, the results show that even in a widely researched field, unexpected pathways can still be revealed.





