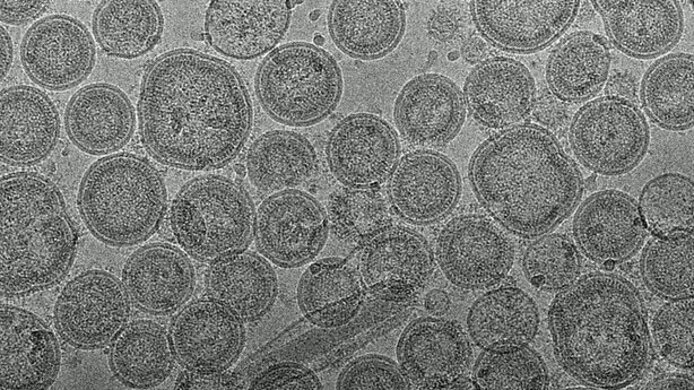
Dual pathogen attack
According to estimates, around 50 million people died from the Spanish flu that broke out in 1918. This pandemic was triggered by a highly infectious strain of the influenza virus. Based on historical and clinical data, we know today that in a large proportion of cases the cause of death was not the viral disease itself, but pneumonia as a result of bacterial infection. Such secondary or superinfections develop on the hotbed of a previous viral infection and are still reason for concern even in the era of antibiotics. Scientists estimate that about a quarter of those who died in the 2009 influenza pandemic (popularly known as “swine flu”), had a bacterial co-infection. Researchers at the Vienna University of Natural Resources and Applied Life Sciences (BOKU) have now been able to show that an influenza vaccination also provides a protective shield against secondary bacterial infections. A research group led by Reingard Grabherr and principal investigator Miriam Klausberger addressed the issue in a project entitled “A VLP approach to combat post-influenza bacterial infections”. The group’s research relied on a promising state-of-the-art vaccine format.
Viruses make it easier to get started
“An influenza infection creates conditions in the body that make it easier for bacteria to get a foothold,” explains Miriam Klausberger. The effect is particularly strong in the first three to seven days after the viral infection. In this time window, even a low dose of bacteria can trigger a severe infection that would otherwise be harmless. A weakened immune system is not the only explanation, however. “After viral infection, the immune response misses important components. Moreover, in order to combat the virus the immune system activates components that are counterproductive in the face of bacterial infection,” notes Klausberger. These components include messenger substances of the immune system, such as type I (α/β) and type II (γ) interferon. They provide protection against viral infections but impair the recruitment and functioning of certain immune cells that are necessary to combat bacterial infection effectively.
Secondary bacterial infections are known not only from influenza but also from rhinoviruses, respiratory syncytial virus (RSV) or SARS-CoV-2. “When viruses multiply in the respiratory tract, they damage the surface of the outer cell layer,” Klausberger describes. “This makes it easier for bacteria to adhere to the mucosa and multiply.” In the influenza pandemics mentioned at the beginning, it was in particular the pathogens Staphylococcus aureus and Streptococcus pneumoniae in that took advantage of the viral leg-up. Both bacteria frequently colonize the human respiratory tract under normal circumstances without causing illness. S. pneumoniae, for example, can be detected in one out of two children up to the age of two. “Knowing that such bacteria are already present means that an influenza infection is an even greater potential hazard,” Klausberger warns.
How effective is vaccine protection?
The idea underlying Klausberger’s project is simple: “We know that an influenza infection prepares the ground for bacterial complications. It may therefore be beneficial to prevent the viral infection through vaccination.” In order to explore this hypothesis, BOKU researchers cooperated with the Moscow-based Mechnikov Research Institute for Vaccines and Sera. The Austrian researchers produced an influenza vaccine based on “virus-like particles” (VLPs). At the Russian institute these VLPs were used to immunize mice for the planned series of experiments.
“VLPs are a phenomenon that occurs naturally in many viruses,” Klausberger explains. “They are defective particles that are produced during virus replication.” While outwardly indistinguishable from their infectious counterparts, they cannot replicate because they lack the necessary genetic material. “This makes VLPs a relatively safe and very effective vaccine format.”
The project “A VLP approach to combat post-influenza bacterial infections” was awarded EUR 358,081 in funding by by the Austrian Science Fund FWF.
The experiment included the vaccinated animals and a control group of unvaccinated mice. In all animals, the first step involved primary infection with an influenza virus. Five days later, the mice were additionally infected with S. aureus or S. pneumoniae. In the course of the experiment, the researchers monitored the success of the vaccination and the course of the disease by studying various parameters, such as the antibody titers detectable in the blood, the spread of the viruses and bacteria in the lung tissue, and the number of deaths.
100 percent survival rate
After a total of 18 days, only ten percent of the unvaccinated control animals had survived the secondary infection with S. aureus, whereas the survival rate in the animals that had received a vaccination adapted to the influenza strain was 100 percent. In the case of S. pneumoniae, none of the unvaccinated animals survived both infections, whereas 60 to 70 percent of the vaccinated animals did.
“Our results provide confirmation that good immunity against influenza viruses can also lower the risk of complications from secondary infections,” Klausberger concludes. The researchers also benefited from gaining insights into the production and purification of VLP-based vaccines. “VLPs have great future potential for vaccine development because they are very effective even at low vaccine doses. Together with other groups at BOKU we are focusing on optimizing the production of such vaccines,” Klausberger said. VLP-based vaccines already exist for human papillomavirus (HPV) and hepatitis B virus. Preparations against influenza viruses are currently investigated in clinical trials.
Personal details
Miriam Klausberger studied biotechnology in Vienna and was a visiting researcher in Singapore, New York and Stockholm. At the Vienna University of Natural Resources and Applied Life Sciences (BOKU), she holds a position as senior scientist in the research group led by Reingard Grabherr, head of the Institute of Molecular Biotechnology. Carried out between 2018 and 2022 in cooperation with the Moscow Mechnikov Research Institute, the project “A VLP approach to combat post-influenza bacterial infections” was awarded EUR 358,081 in funding by by the Austrian Science Fund FWF.
Publications
Klausberger M., Leneva I.A., Egorov A., Strobl F. et al.: Off-target effects of an insect cell-expressed influenza HA-pseudotyped Gag-VLP preparation in limiting postinfluenza Staphylococcus aureus infections, in: Vaccine 38(4), Jan. 2020
Klausberger M., Leneva I.A., Falynskova I.N., Vasiliev K. et al.: The Potential of Influenza HA-Specific Immunity in Mitigating Lethality of Postinfluenza Pneumococcal Infections, in: Vaccines (Basel) 7(4), Nov. 2019