Messenger substance live on show
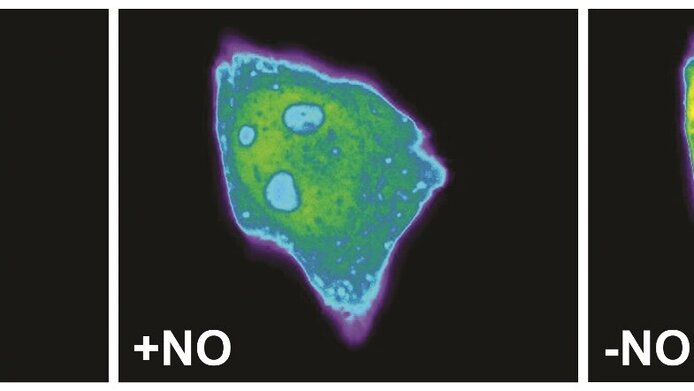
Since the discovery of its role in erectile dysfunction, it went upwards – with the recognition of nitric oxide's (NO) role as a regulator of important functions in the cardiovascular, nervous and immune systems. Up to now, however, it was not possible to measure its cellular concentration directly in real time. With the help of funding from the Austrian Science Fund FWF, a research group led by Roland Malli at the Institute of Molecular Biology and Biochemistry of the Medical University of Graz has now succeeded in accomplishing this.
Fluorescent protein
The team produced a fluorescent protein whose fluorescence changes when it binds to NO. The change in the fluorescence depends on the concentration of the NO; it is also reversible and the process more or less unfolds in real time. Consequently, it fulfils all of the conditions necessary to enable intracellular measurement of the NO dynamics. Malli and his team succeeded in measuring these dynamics in different types of cells. These were genetically modified in such a way that they produced the new protein themselves and the NO concentration could be measured on the basis of the fluorescence emitted by the cells.
The chimera
Proteins that bind to NO with a high level of specificity were crucial to the group's research. "We considered fusing this kind of NO-binding protein to a naturally fluorescent protein. In other words, to create a chimeric new protein that would be able to both bind to NO and fluoresce", explains Malli. The assumption was that the fluorescence of the new protein would change when it binds to the NO. Computer simulations confirmed that this was the case for a very special combination of proteins.
Playing around with proteins
Based on the simulation, Malli started by making a number of new proteins. The NO-binding component in all of these proteins came from a bacterial protein called NorR. This is a so-called transcription factor, which acts as an intermediary between an NO signal and the activation of genetically stored information. Malli explains the advantage of using a bacterial protein as follows: "It was very likely that this would have little influence on mammalian cells, in which we wanted to measure the NO." The team then merged the part of the NorR, which is specifically responsible for the NO binding, with five different fluorescent proteins. The fusion proteins, which were referred to as "genetically encoded NO probes" (geNOps), were actually produced in such a way that their genetic information, which was rapidly introduced into cells, resulted in the biosynthesis of functional NO probes.
Less is more
Malli himself was surprised at how effective the different geNOps were: "As expected, the binding of the NO resulted in a reduction – that is quenching – of the fluorescence. The effect can be clearly measured and its intensity is depends directly on the NO concentration. Moreover, the NO binding is easy to reverse which means that changes in the cellular NO concentration can be measured very quickly. This is a truly fantastic advance!"
Patented technology
Based on this, Malli and his team were able to measure the NO concentration in cellular compartments like the mitochondria. In addition, they combined the use of geNOps with the measurement of the calcium concentration in cells and, in this way, were able to demonstrate links between the activity of the messenger substances NO and calcium at a high temporal resolution. The development of the geNOPs, which has already been patented and was made possible by the support of the FWF, heralds a new dawn in the measurement of nitric oxide and its wide-ranging physiological impacts on the level of individual cells.
Personal details Roland Malli carries out research at the Institute of Molecular Biology and Biochemistry at the Medical University of Graz and focuses in particular on the measurement of intracellular ions and their role as signalling substances. In addition to electrophysiological methods, he has developed microscopic processes and received multiple awards for his patented developments in the past year.
Original publication





