How to simulate protein recognition
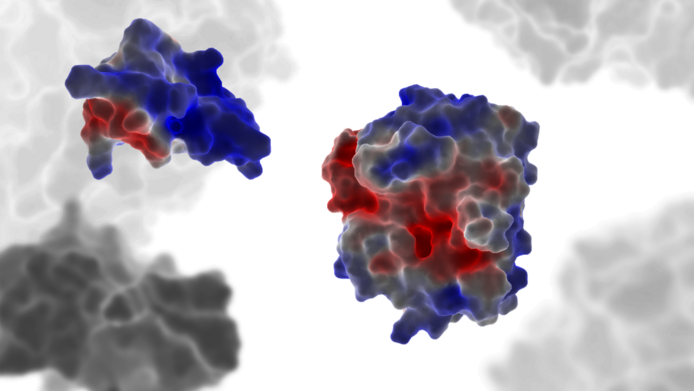
Enzymes are among the most important tools of the animate natural world. These small biocatalysts accelerate various transformation processes and are found in every organism and every cell, where they are involved, amongst other things, in the body's energy production. So-called proteases are also counted as enzymes. They are specialised in breaking down proteins. In the digestive system, they break down all the protein components of the incoming food and prepare them for utilisation. Very similar proteases are also involved in the body's blood clotting function. As a component of the clotting cascade, they are highly specialised elements of a complex, self-regulating system.
Although almost identical in structure, different types of proteases can fulfil greatly differing functions. Some are very specific and only address one particular protein, others are “promiscuous”, which means they are almost universal splitting tools that break down just about any protein they come across – an ability that is required for digestion, for example. Together with his team, Klaus R. Liedl, head of the Institute for General, Inorganic and Theoretical Chemistry at the University of Innsbruck, is exploring these different properties of proteases. The researchers are using sophisticated computer simulations to find out why the protein tools behave so differently despite their almost identical structure.
Improvement of therapeutic antibodies
Once we are able to better understand and predict which proteases function very specifically and which are universal, we can apply this knowledge immunology and therapy, for instance in immunisation and the therapy of viral diseases such as infections with HIV, influenza or coronaviruses, which are constantly changing through mutation. “For us, the proteases are basically just a test system that helps us better understand the physical processes involved in the recognition of biomolecules of all kinds,” the scientist explains. “Transferring the findings to the protein structures of therapeutic antibodies, for instance, could greatly improve the targeted effect of these biopharmaceuticals.” Researchers put great hopes in active substances of this kind with a view to many diagnostic and therapeutic areas – first and foremost in the fight against cancer.
The proteases were chosen as the subject of research because there is a particularly good body of data about them from past drug research – including X-ray structure analyses and entire databases on the behaviour of a wide range of variants. According to previous findings, the differences between specific and promiscuous proteases lie mainly in their dynamic properties, which means they only become apparent as soon as the biomolecules can be recorded in motion. This is where the simulation tools developed by Liedl and his team come into play. They are designed to calculate the behaviour of the proteases over as long a period of time as possible.
Bridging widely divergent time scales
“The crux is to watch the proteases for as long as possible,” summarises Liedl. But herein precisely lies the major challenge for the researchers. “The problem is that the time scales of the simulation and the phenomenon we want to look at are very far apart: the simulated behaviour of the atoms involved takes place on a femtosecond time scale, i.e. in millionths of billionths of a second, while the phenomena we are interested in occur in the millisecond range, i.e., involving a thousandth of a second.” To illustrate the difference: this is like trying to predict the behaviour of an object that moves every second over a time span of at least 32,000 years.
For this reason, one of the most difficult tasks in this type of research is providing the necessary computing power to complete such a simulation in a manageable time frame. “We work with a combination of local computer infrastructure, which we set up and programme ourselves, and university computing centres,” notes Liedl. The research group is also testing the use of artificial intelligence systems in order to obtain the desired results more efficiently.
As researchers gain a better understanding of how the dynamics and structure of proteases are linked, they also come closer to the vision of specifically designing the recognition characteristics of biomolecules. In the case of therapeutic antibodies, they could be specifically adapted by changing the genetics of their production system – certain cell lines that produce the desired proteins. Liedl: “In practice, we would propose mutations of certain gene sequences that change the properties of the therapeutic antibodies in the desired direction and make them more promiscuous, more specific or more durable.”
Personal details
Klaus R. Liedl is Professor of Theoretical Chemistry and Head of the Institute for General, Inorganic and Theoretical Chemistry at the University of Innsbruck. Holding degrees in chemistry, mathematics and law, Liedl has also taught at universities in Italy, Brazil, Thailand and Indonesia. “Characterisation of Promiscuity and Specificity in Proteases” is Liedl's seventh FWF project, which is funded with EUR 393,000 and will run until 2022.
Publications
Kahler U, Kamenik AS, Waibl F, Kraml J, Liedl KR: Protein-Protein Binding as a Two-Step Mechanism: Preselection of Encounter Poses during the Binding of BPTI and Trypsin, in: Biophysical Journal 2020
Hofer F, Kraml J, Kahler U, Kamenik AS, Liedl KR: Catalytic Site pKa Values of Aspartic, Cysteine, and Serine Proteases: Constant pH MD Simulations, in: Journal of Chemical Information and Modeling 2020
Kahler U, Kamenik AS, Kraml J, Liedl KR: Sodium-induced population shift drives activation of thrombin, in: Scientific Reports 2020
Guthmiller J, Yu-LingLan L, Fernández-Quintero ML, Han J et al.: Polyreactive Broadly Neutralizing B cells Are Selected to Provide Defense against Pandemic Threat Influenza Viruses, in: Immunity 2020
Fernández-Quintero ML, Loeffler JR, Bacher LM, Waibl F et al.: Local and Global Rigidification Upon Antibody Affinity Maturation, in: Frontiers in Molecular Biosciences 2020





