Hope for a New Osteoporosis Treatment

From about middle age, many people have to grapple with the effects of osteoporosis. The condition, which slows down the creation of new bone, leading to brittle bones and diminished bone density, doesn’t seem to cause any symptoms in the early stages. However, the risk of suffering a fracture is significantly increased. In severely affected people, even simple, everyday movements can lead to fractured vertebrae or a broken femur or wrist.
Osteoporosis is a disease typically associated with age and is twice as common in women as it is in men. Many of the physical changes induced by aging are bad news for our bones: be it the lowered sex hormone levels, vitamin D deficiency or the shorter telomers, whose job it is to protect our genetic material. As our body undergoes such age-related transformations, it becomes more prone to inflammation reactions – a phenomenon referred to as “inflammaging”, which is also associated with a higher risk of osteoporosis.
Testing medication for treating rheumatism
In a project funded by the FWF, Peter Pietschmann and his team at the Center for Pathophysiology, Infectiology and Immunology at the Medical University of Vienna investigate the ways in which the human immune system interacts with bone development. The research group focuses on an anti-inflammatory agent called Baricitinib, which is already used in the treatment of inflamed joints as a symptom of rheumatoid arthritis.
The medical researchers are eager to find out whether the drug could be beneficial in treating osteoporosis. “A German research group was able to show in an animal model that Baricitinib has a positive effect on bone metabolism in young organisms,” Pietschmann explains. “We are now investigating whether this effect can be reproduced in a model for age-related osteoporosis.”
The physician Peter Pietschmann is investigating the biology and pathophysiology of bones using molecular, cellular and translational approaches. A new approach using an established active agent for the treatment of rheumatoid arthritis is intended to open up new therapeutic options.
Putting a stop to inflammatory mediators
Baricitinib acts on so-called proinflammatory cytokines. These are proteins produced by cells in many parts of the body, which play a mediating role in immune reactions. They are activated when the body detects a viral disease or damaged tissue and also in inflammations affecting the whole body, which become more common as we age. They also play a part in the development of osteoporosis. Baricitinib and other so-called JAK inhibitors suppress the development of such cytokines and interrupt their signal pathways, thus regulating inflammation reactions.
To find out whether this kind of immunosuppression will also work for age-related bone loss, the researchers are relying on a special mouse model. Mice belonging to the SAMP8 line age particularly fast, which makes them an important model for research on osteoporosis and gerontological diseases in general. In their project, Pietschmann and his team treated a total of 60 female mice with Baricitinib or a control substance for six weeks.
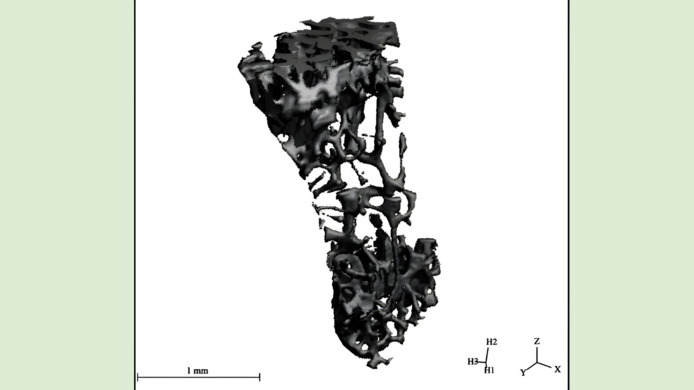
In a subsequent step, the animals were subjected to a variety of tests. The researchers checked for inflammation reactions in the mice, employed radiological and microscopic aids to explore the inside of their bones and looked for various markers, which can be used to measure changes in bone metabolism. While data analysis is ongoing in this project, which will run through 2025, the team can already present first results.
Improved bone structure
“For one thing, our analysis of the cytokine production shows that administering the active ingredient lowers inflammation markers in the mice’ bodies. For another thing, a histomorphometry, a computer-assisted analysis of thin layers of tissue using a microscope, has produced evidence of a positive change in bone structures,” Pietschmann explains. “The observed effect was less pronounced in a micro computed tomography of the bone. This could be due to the fact that the microscopic method and the micro-CT measure different bone properties.”
A method employed in molecular biology, an analysis of RNA, which plays an important part in transferring genetic information for building proteins, could produce further insights. In bioinformatic approaches, the data gained through RNA sequencing can be used to observe whether the agent is effective on the level of gene expression. This enables researchers to gain a comprehensive picture of the molecular-biological processes at play.
The results gained so far do not yet conclusively indicate whether Baricitinib will offer an effective treatment for osteoporosis. But even if the team succeeds in achieving a proof of concept in the mouse model, the admission process would take a while. “The clinical phase would be less troublesome because the drug has already been approved for the treatment of other conditions,” Pietschmann explains. “In order to use it as an osteoporosis drug, however, we would have to prove that typical fractures would be greatly reduced compared to a control group – which would take several years in any case.”
Personal details
Peter Pietschmann is the Head of the Division of Cellular and Molecular Pathophysiology at the Medical University of Vienna’s Center for Pathophysiology, Infectiology and Immunology. Together with his research group, the physician is currently investigating the biology and pathophysiology of bones using molecular, cellular and translational approaches. Since 2023, Pietschmann has served as the President of the European Calcified Tissue Society (ECTS), a leading bone research institution in Europe. The “Baricitinib for the treatment of age-related osteoporosis” project, which runs from 2022 to 2025, has been endowed with EUR 241,000 by the Austrian Science Fund (FWF).
Publications
Meshcheryakova A., Bohdan S., Zimmermann Ph., Jaritz M., Pietschmann P., Mechtcheriakova D.: RNA-Binding Proteins as Novel Effectors in Osteoblasts and Osteoclasts: A Systems Biology Approach to Dissect the Transcriptional Landscape, in: International Journal of Molecular Sciences 2024
Pietschmann P., Butylina M., Kerschan-Schindl K., Sipos W.: Mechanisms of Systemic Osteoporosis in Rheumatoid Arthritis, in: International Journal of Molecular Sciences 2022
Kerschan-Schindl K., Hackl M., Boschitsch E. et al.: Diagnostic Performance of a Panel of miRNAs (OsteomiR) for Osteoporosis in a Cohort of Postmenopausal Women, in: Calcified Tissue International 2021