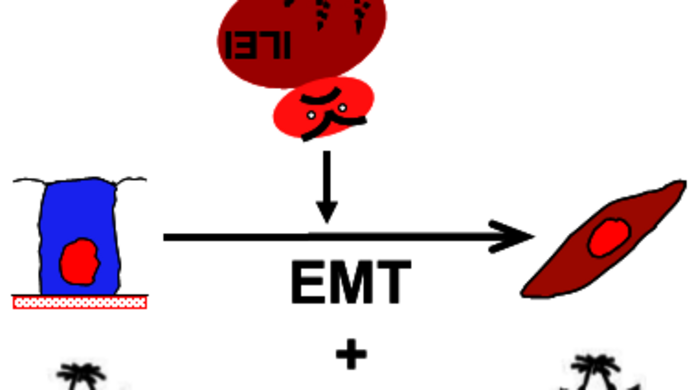
A power couple mobilises cancer cells

When Agnes Csiszar talks about the FAM3 protein family, one is reminded of a mafia clan whose members are involved in various diseases but are difficult to catch. The FAM3C or ILEI protein is considered to be an important signal inducer for the metastasis of cancer cells. In a process called EMT (epithelial-to-mesenchymal transition), ILEI is involved in mobilising cells that are immobile and connected in solid tumours, thereby enabling the cancer to spread throughout the body. Other members of FAM3 (A, B and D) are considered potentially important molecules for the treatment of other common diseases.
From protein pack to messenger substance
The structure of substances in and around a cell is not activated at the push of a button. There are always carefully regulated signalling pathways and molecular cascades that set processes in motion. Agnes Csiszar spent several years at the Institute of Molecular Pathology (IMP) in Vienna investigating ILEI and compiling the biochemical components of its profile. It was already known that the FAM3C protein remains concentrated at one site in non-metastatic cancer cells, as this could be demonstrated by targeted histochemical staining. In metastasizing cancer cells, however, ILEI is distributed over the entire cell. Csiszar has shown that metastatic cancer cells secrete much more ILEI and that the amount correlates with the level of metastasis. Though, it had remained unknown how the released ILEI is further regulated and converted into an active form. The research of Agnes Csiszar at the Institute for Cancer Research at the Medical University of Vienna was supported by the Austrian Science Fund FWF. In her standalone project, Mechanism and role of ILEI dimerisation in tumour progression, she has now succeeded in learning more about the way of ILEI signalling that ultimately promotes metastasis.
A date in extracellular space
Once ILEI has been released by the cells, the individual proteins (monomers) look for an identical partner molecule in the extracellular space and form active power pairs (dimers): “In the two ILEI monomers, those chemical bonds that keep the molecule in shape are disrupted and reconnected to their identical counterpart. This bond is required in order to activate ILEI and spread cancer”, explains Agnes Csiszar.
The molecular biologist obtained functional results from cell cultures, from mouse models and through the use of normal and dimerization-defective ILEI molecules. In cooperation with Astra Zeneca, these data were confirmed through crystal structure investigations of the molecule. The pharmaceutical company had previously published about FAM3B, which is regarded as a promising target for diabetes drugs. Agnes Csiszar contacted the research department, and they teamed up and revealed how the two ILEI monomers can bind to each other. In follow-up projects, Agnes Csiszar is now investigating which factors accelerate the formation of such power couples (dimers) and which receptor in the signal pathway is activated by the ILEI pair. Both the highly active signalling molecules and the receptors would be suitable therapeutic targets. However, the latter still have to be identified.
Personal details Agnes Csiszar studied molecular biology in Hungary and obtained her PhD at the University of Cologne. Since 2014, she has been an associate group leader at the Institute for Cancer Research at the Medical University of Vienna. She is interested in cancer metastasis, particularly in key molecules and regulatory mechanisms of epithelial mesenchymal transition (EMT) and the regulation of growth factor activity.
Publications