“The most important thing is to encourage curiosity”

Mr. Winter, our cells need proteins as building blocks. In your research you are not, however, concerned with their production, but with their degradation. Why is that important?
Georg Winter: In biological contexts, the degradation of building blocks is always relevant. Our cells are the venue of a constant synthesis and elimination of fundamental elements. As one of the important building blocks, proteins are degraded in different ways. Some of the mechanisms are strictly regulated in order to be able, for example, to respond to environmental influences. Others operate in the background as quality control mechanisms, for instance when proteins have been produced incorrectly. All these processes are important for keeping cells intact. If degradation is blocked or if the balance is upset by mutations, this will often cause disease, including cancers such as leukemia. Research into so-called “degraders” makes it possible for us to eliminate proteins in a targeted manner, including those that are needed by cancer cells to grow and divide.
How does this mechanism work?
Winter: Basically, the idea is to bring two interaction partners close together: the protein we want removed and the cellular machinery for protein degradation, specifically the so-called E3 ubiquitin ligases. These ligases can then mark the target protein for degradation by attaching to it another, smaller protein called ubiquitin. You can picture it as though the E3 ligases were sticking post-its on the protein that needs to be removed. Once a protein has been marked with three ubiquitins in series – has collected three post-its, if you like – that is the signal for the cell to start degradation. For this reason, ubiquitination is also known as the “kiss of death”. With the degraders, we are pursuing the goal of attaching these markers specifically to disease-causing proteins.
What do such degraders look like?
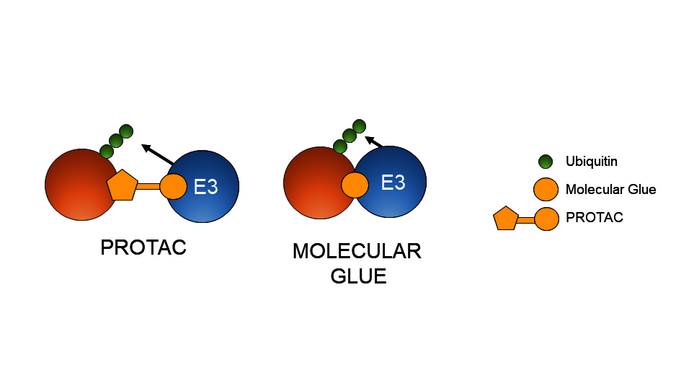
Winter: There are different ways of designing the small molecules that function as degraders. Roughly we can distinguish two groups. One are the so-called PROTACs, which I worked on during my stay as a postdoc in the USA. In their case, the bond between the E3 ligase and the target protein is relatively simple: PROTACs have one binding site for each of the two interacting partners, connected by a linker. In this way they can bind simultaneously to both the target protein and the degradation machinery.
In the case of Proxygen, however, we are interested in the other group of so-called “molecular glue degraders”, or molecular glues for short. Their design is a little more complicated because they bind to only one of the two partners and change its surface in the process. If their architecture is designed in the right way, they change the binding site in such a way that the second protein is attracted to it.
Why are molecular glues more interesting from your point of view?
Winter: For one thing, they are smaller molecules and therefore more suitable for producing orally administered drugs. Secondly, we don't have to create a specific binding site for the target protein on them. Biologically speaking, that is exciting. We often think of proteins as small spheres with indentations. In reality, however, many proteins are more like long spaghetti made of unfolded chains of amino acids. In the case of such proteins, it is difficult to find a domain to which PROTACs or classical drug types, such as inhibitors, can bind. Given that molecular glues do not need such a specific binding site, they allow for a much larger range of target proteins. That is their strength.
Proxygen was founded in 2020 as a consequence of these considerations. What exactly is the idea behind the company?
Winter: At Proxygen, we are trying to close the gap to proteins that simply cannot be targeted with conventional drugs. Unlike others, our approach is not geared to reversing individual causes of disease. Instead, we aim to reprogram the mechanism of protein degradation via a chemical route. In order to do that, we are streamlining the strategies used to identify molecular glues, because such molecules have traditionally only been discovered by chance.
To what extent has basic research contributed to the concept of Proxygen?
Winter: Our scientific projects have given us important insights into the different types of degraders. Also, we have discovered quick ways to find and characterize molecular glues. In 2019, we were able to show in a publication that there is a genetic switch that can turn on or off 300 of the total number of about 600 E3 ligases at once. In 2020 we published a paper describing how this mechanism can be used to search for molecular glues.
We quickly realized that this approach had breakthrough potential, but that we would not be able to put it to good use in a purely academic setting. It has since evolved into one of the methods we use at Proxygen today. However, the process is not as linear as it may now seem to be. The core concepts that we apply at Proxygen are rather indirect outcrops from our scientific projects – and I believe that anything else would not be right. Basic research should not be conducted with the intention of turning it into an economic enterprise.
What role can funding play, as provided by the FWF?
Winter: The FWF grants were essential for me at the outset to enable me to start my lab and then upscale it. They were the most important grants I have received because they were the first ones. I used the research grants to try and understand molecular mechanisms as precisely as possible – and at that time that was not with a view to any possible application. The most important thing is to encourage curiosity. By adding up the learning curves from individual projects, you arrive at a knowledge base on which to build bigger things. If you want to develop drugs, this mechanistic understanding is fundamental.
Another aspect of the grants is that they enable us to foster new talent. Matthias Brand, for instance – the first student I supervised in my lab at CeMM –, is now the co-founder and Chief Scientific Officer of Proxygen. Ultimately, the publications from such projects also enhance our credibility because they testify to the expertise we, the founders of the company, have in our field. This is certainly a major competitive edge that Proxygen has over other companies that take a similar approach.
What impact do investments like MSD's have on Vienna and Austria as a research location?
Winter: Such cooperation projects are beneficial because they show that Austrian companies can grow beyond the local biotope and become international players. The impact is particularly noticeable when recruiting new staff. There are talented young people in Vienna, but many are still inexperienced in the biotech industry. Compared to hotspots such as Boston, San Francisco or Basel, we find it more difficult to get experienced people. With every successful model that is evolves in Vienna or Austria, the location becomes more attractive for competent people to take the chance of relocating here.
What does the collaboration with MSD mean for Proxygen?
Winter: In ourcase, this is the third collaboration with a pharmaceutical company – after having worked with Boehringer Ingelheim and Merck in Germany. Such cooperation projects are preceded by a long process in which the start-up and the people behind it are closely scrutinized. In this light, it is a great honor for Proxygen when global players decide to cooperate with us, and it does fill us with pride. But we must not forget that this is only the beginning of the joint project. Now we have to prove our worth as partners. Our field is very competitive globally and is currently enjoying a major upswing. And that means, we must continue to come up with new ideas in order to remain one of the most innovative companies.
Georg Winter acquired his PhD from the CeMM Research Center for Molecular Medicine in Vienna before moving to Harvard Medical School in Boston as a postdoctoral fellow in 2013. Having returned to CeMM as a Principial Investigator in 2016, he cofounded the biotech start-up Proxygen with Matthias Brand, Stefan Kubicek and Giulio Superti-Furga in 2020. In April 2023, Proxygen announced a collaboration with the U.S. pharmaceutical company MSD, one of the largest drug manufacturers in the world, with an investment of around 2.3 billion euros.





